 | . | Step 16 of 41 |
 |
Clinical Stem 3
A patient with right upper lobe adenocarcinoma progressing on biomarker-directed therapy
A patient with right upper lobe adenocarcinoma progressing on biomarker-directed therapy
Answer D
Molecular testing is currently indicated in NSCLC showing an adenocarcinoma component regardless of histologic grade or subtype, in small biopsies or incomplete excision specimens showing only squamous or small cell histology, and in poorly differentiated tumors or tumors that otherwise cannot be classified as pure squamous, pure small cell, or pure neuroendocrine carcinomas. Given the emergence of new molecular markers for other histologic types, the need for specific tests should be clarified with the referring oncologist prior to tissue acquisition.
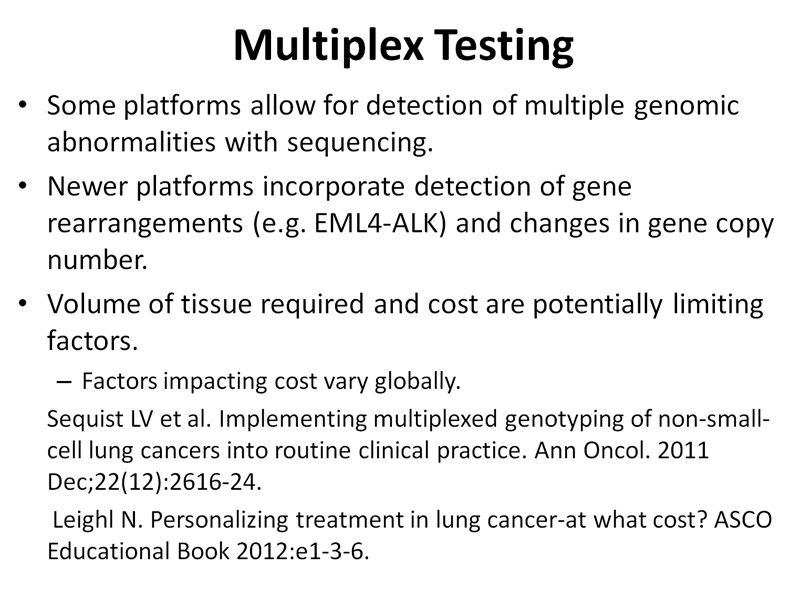
Each molecular laboratory has a minimal amount and concentration of tumor cells required for accurate detection of molecular alterations based on specific tumor enrichment protocols available and assay platforms used for testing. The treating team and the pathologist should be aware of these requirements so that the number of specimens rejected by the molecular laboratory is minimized.
Click here to download supplement materials (1)
Click here to download supplement materials (2)
References:
- Shiau CJ, BabwahJ, daCunhaSantosG, et al. Selection of Samples for Epidermal Growth Factor Receptor Mutation Analysis in Non-squamous Non-small cell lung carcinoma. Modern Pathol 2012; 25 (suppl2): 490A (Abstract 2045).
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013 Apr 2. [Epub ahead of print] PubMed PMID: 23552377.







