 | . | Step 35 of 40 |
 |
Clinical Stem 2
A patient with pulmonary nodules 1 year after curative intent resection of primary lung adenocarcinoma
A patient with pulmonary nodules 1 year after curative intent resection of primary lung adenocarcinoma
Answer A
Documenting results of molecular tests on body fluids is increasingly relevant. Studies show that cell blocks from pleural or pericardial fluid are adequate for EGFR and KRAS mutation analysis. Similar to fine needle aspirates from other sites, pleural fluid cytology specimens qualifying for molecular analysis should contain at least 40% tumor cells. One semi-quantitative estimate of specimen cellularity describes sparse cellularity as less than 300 tumor cells, low cellularity as 300-1000 tumor cells, and normal cellularity as more than 1000 tumor cells. Specimens with sparse cellularity were shown to have a high PCR failure rate due to poor quality, or insufficient quantity of DNA for EGFR and KRAS mutation analysis.
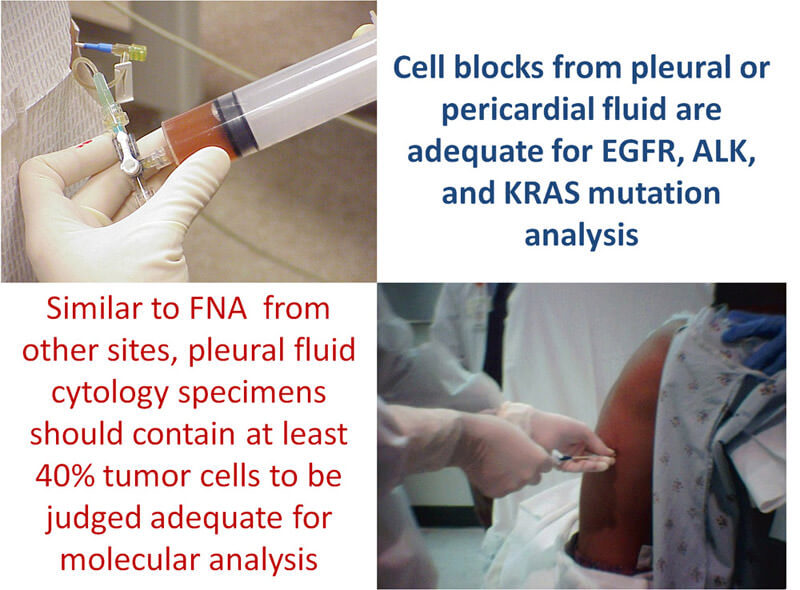
Pleural and pericardial fluids are inadequate for molecular testing in almost 4% of cases, which is less than the 7.5% insufficiency rate noted in CT-guided fine needle aspirates.
References:- Billah S, Stewart J, Staerkel G, et al. EGFR and KRAS mutations in lung carcinoma: molecular testing by using cytology specimens. Cancer Cytopathol. 2011 ;119:111-7.
- Rekhtman N, Brandt SM, Sigel CS, et al. Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing. J Thorac Oncol. 2011;6:451-8.
- Aisner DL, Marshall CB. Molecular pathology of non-small cell lung cancer: a practical guide. Am J Clin Pathol. 2012; 138: 332-46.







