 | . | Step 18 of 41 |
 |
Clinical Stem 1
Non-smoking female with a new right upper lobe lung mass and three PET-positive mediastinal lymph nodes
Non-smoking female with a new right upper lobe lung mass and three PET-positive mediastinal lymph nodes
Answer D
KRAS mutation occurs in approximately 15?30% of NSCLC, mostly in lung adenocarcinoma, rarely in squamous cell carcinoma and is mutually exclusive with EGFR mutations. Its presence confers resistance to treatment with TKIs. Clinically relevant mutations found in 3.5?7% of EBUS-TBNA specimens can be detected by RT-PCR or DNA sequencing. The optimal methodology for the detection of KRAS mutation for needle biopsied samples is uncertain at this time; in fact, needle specimens may be inadequate compared with resected specimens which show the mutation at higher frequencies.
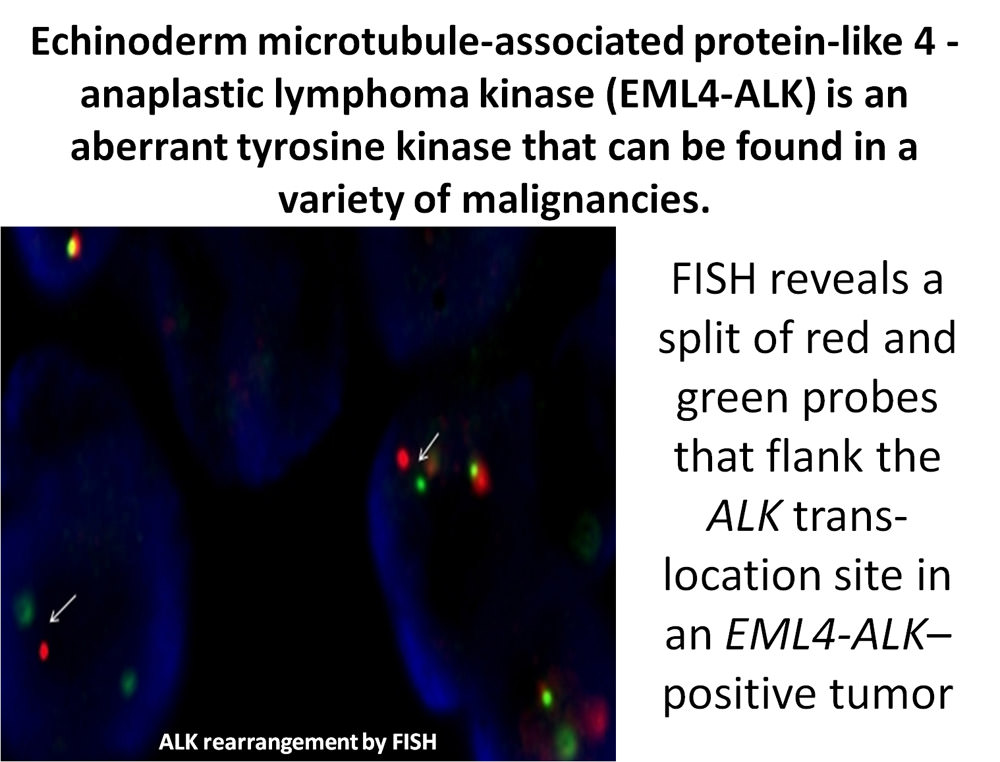
Approximately 3-7% of NSCLC harbor anaplastic lymphoma kinase (ALK) fusions and in the vast majority of cases, ALK rearrangements are non-overlapping with other oncogenic mutations such as EGFR and KRAS mutations found in NSCLC. ALK is a receptor tyrosine kinase that is aberrant in a variety of malignancies. The EML4-ALK fusion oncogene results from a small inversion within chromosome 2p. This leads to expression of a chimeric tyrosine kinase, in which the N-terminal half of echinoderm microtubule-associated protein-like 4, also known as EML4, is fused to the intracellular kinase domain of ALK. Clinically, the presence of ALK fusions is associated with EGFR TKI resistance, ALK gene rearrangement predicts response to inhibitors of the chimeric tyrosine kinase synthesized by this oncogene and could therefore assist in the management of this patient.
EGFR is a growth promoting protein located within the cytoplasmic membrane. Its external domain binds growth factors and is the target of monoclonal antibody drugs while the internal domain, including the tyrosine kinase domain, is the target of small molecule drugs, known as TKIs. Fine needle aspirates, unstained slides and formalin-fixed, paraffin-embedded tissues, when core tissue is obtained, can be sent for EGFR mutation analysis or investigation of increased gene copy number.
An established test for ALK gene rearrangement is fluorescence in situ hybridization, also known as FISH. Some molecular laboratories require at least 2 mL of special media for fine needle aspirates or 10% neutral buffered FFPE tissue; ALK gene rearrangement can also be detected by immunohistochemistry, PCR and DNA sequencing, and is feasible in EBUS-TBNA specimens.
References:An established test for ALK gene rearrangement is fluorescence in situ hybridization, also known as FISH. Some molecular laboratories require at least 2 mL of special media for fine needle aspirates or 10% neutral buffered FFPE tissue; ALK gene rearrangement can also be detected by immunohistochemistry, PCR and DNA sequencing, and is feasible in EBUS-TBNA specimens.
- Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958-67.
- Rosell R, Gervais R, Vergnenegre A, et al. Erlotinib versus chemotherapy (CT) in advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: Interim results of the European Erlotinib Versus Chemotherapy (EURTAC) phase III randomized trial. J Clin Oncol 29: 2011 (suppl; abstr 7503).
- http://www.mycancergenome.org/molecular-pathology (accessed August 2011).
- Nakajima T, Yasufuku K, Nakagawara A, et al. Multi-gene mutation analysis of metastatic lymph nodes in non-small cell lung cancer diagnosed by EBUS-TBNA. Chest 2011;12:293-297.
- Sakairi Y, Nakajima T, Yasufuku K, et al. EML4-ALK fusion gene assessment using metastatic lymph node samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Clin Cancer Res. 2010;16:4938-45.







